The Hemp Advancement Act and the 167:1 Problem
The outrageous federal cannabis policy you should know about but probably don't
Representative Pingree (D-ME) filed the Hemp Advancement Act of 2022 last week. I go through the bill section by section below and attempt to translate into plain English.
If you want to go straight to the juicy stuff, skip to the end where I discuss Section 9. That section blesses an unlawful DEA regulation that hands district courts unfettered discretion to increase the total amount of marijuana attributable to a criminal defendant by a factor of 167.
Section 1
This is the bill’s “short title.”
Section 2
Section 2 is important. It amends the definition of “hemp” from the 2018 Farm Bill and defines two new terms: “hemp product” and “total tetrahydrocannabinol concentration.”
New definition of “hemp.” Section 2 amends the 2018 Farm Bill’s definition of hemp in four ways. First, raises the THC concentration threshold from .3% to 1%. Second it shifts the focus from delta-9 THC to “total tetrahydrocannabinols concentration”—a term defined later in the same section and discussed further below. Third, it includes “hemp extract” within the definition of “hemp,” so long as the hemp extract is
used in the making of a hemp product (another term defined later in section 2 and discussed further below)
hasn’t been packaged as a finished product
isn’t intended for sale to consumer
has a total THC concentration in excess of 1% on a dry weight basis AND
is stored, transported, and processed in accordance with section 297F (section 7 of the bill, which, as discussed below, imposes various requirements on “in-process hemp extract”)
Fourth and finally, it clarifies that “hemp” cannot be “intended for sale to consumers.” The way the bill is worded, it’s not clear whether this “not intended for sale to consumers” only applies to non-hemp-extract “hemp” or to all “hemp.” My guess is that the prohibition would apply across the board.
Next, section 2 defines “hemp product” to mean a hemp-derived product with a total THC concentration of .3% or less on a dry weight basis:
And finally, whereas the 2018 Farm Bill defined hemp in terms of relative delta-9 THC concentration, the Hemp Advancement Act focuses on “total tetrahydrocannabinol concentration,” a term it defines to mean the aggregate concentration of delta-8, delta-9, and delta-10 THC, plus “the optical isomers of such substances.”
When you put all this together, these changes would effectively lower the THC threshold from its current calibration under the 2018 Farm Bill. That is because under both the 2018 Farm Bill and the Hemp Advancement Act, only hemp products at or below the .3% THC threshold may be sold, but the Hemp Adfancement Act increases the number of compounds that contribute to “total tetrahydrocannabinol concentration.” Consider a hemp product that is .3% delta-9 THC on a dry-weight basis. Under the 2018 Farm Bill, that product would qualify as a marketable “hemp” product. Under the Hemp Advancement Act of 2022, however, that same product would exceed the .3% Total THC Concentration limit if it contained even a trace amount of delta-8, delta-10, or a relevant optical isomer.
Section 3
The 2018 Farm Bill permitted states and tribes to submit plans to govern the hemp industry in their jurisdictions to USDA for approval. If USDA approved a plan, it would govern in that jurisdiction. If a state or tribe didn’t submit a plan, or if USDA didn’t approve the plan a state or tribe submitted, a default USDA plan would apply in that jurisdiction.
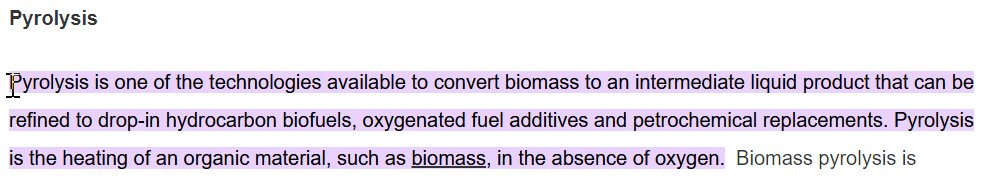
Section 3 amends the 2018 Farm Bill’s requirements for state and tribal plans. Among other things, it requires states and tribes to use USDA-accredited labs to test their hemp and permits them to dispose of hemp through “pyrolysis,” which, according to USDA’s Agricultural Research Service, means this:
Still confused? Here’s a chart that won’t help:
If a hemp producer/processer disposes of hemp through “pyrolysis,” section 3 adds, “products from [that] pyrolysis disposal may enter the stream of commerce.” I suspect (but am not certain) these pyrolisis provisions are designed to encourage the conversion of hemp waste into biofuel to offset fossil-fuel consumption.
Finally, section 3 removes a provision of the 2018 Farm Bill that prohibits those convicted of a drug-related felony in the past 10 years from participating in the hemp industry.
Section 4
Section 4 provides that anyone excluded from participating in the hemp industry under the previously applicable 2018 Farm Bill provisions excluding those convicted of drug-related felonies “shall not be excluded from participation … on these grounds.” In other words, it reinstates their eligibility to participate in the industry going forward.
Section 5
Section 5 takes the amendments Section 3 made to the provisions governing ttate and tribal plans and makes the same changes applicable to the USDA’s default plan.
Section 6
Section 6 puts USDA in charge of hemp testing labs. Under the 2018 Farm Bill, these labs must register with DEA under 21 U.S.C. 823. Why? Because they might possess hot hemp, i.e. “marihuana.” See 21 U.S.C. 844 (making it a crime to merely possess a controlled substance without DEA permission).
Section 7
Section 7 provides that “in-process hemp extract” must
be derived from hemp produced in accordance with the Hemp Advancement Act
be processed further or incorporated into another product
neither be packaged as a hemp product nor sold or offered for sale
not be used to produce a hemp product that exceeds .3% total THC concentration
be produced, stored, transported, and processed in a facility bonded under certain regulations
The second and fourth requirements are a bit confusing. The second—that in-process hemp extract be processed further or incorporated into “another product”—implies that in-process hemp extract could be a “product” even though the third requirement seems to say precisely the opposite. The fourth—that in-process hemp extract not be used to produce a hemp product that exceeds .3% total THC concentration—is even more confusing. According to the definition of “hemp product” in Section 2, there is no such thing as a “hemp product that exceeds .3% total [THC] concentration.”
Section 7 also directs USDA to promulgate regulations to govern “facilities” that handle in-process hemp extract. Those regulations must, among other things, ensure secure transportation and include record keeping requirements.
One final bit of confusion: Does “in-process hemp extract” qualify as “hemp extract” as the term is used in Section 2? Before you answer, be sure to check out my analysis of Section 9 below.
Section 8
Section 8 amends the “note” to 7 U.S.C. 1639o. Currently, the note says that nothing in “this Title” or an amendment to “this Title” prohibits the interstate commerce of hemp as defined in section 1639o “or hemp products.” Section 8 would add that nothing in “this Title” or an amendment to “this Title” “permits interstate commerce of products containing cannabinoids that are not naturally occurring in the plant Cannabis sativa L. or that are manufactured by means of chemical synthesis.”
This provision closes the door on interstate commerce of delta-8 products that are made through chemical synthesis (which, I understand, is most of them). It presumably would permit the use of extraction to derive “tinctures.”
Hard to know how “naturally occurring” and “manufactured by means of chemical synthesis” would be construed by courts and regulators. Congress really should either define terms like these or stop using them.
Section 9
Section 9 amends the Controlled Substances Act’s definition of “marihuana” and its listing of “Tetrahydrocannabinols” in schedule I. “Marihuana” is amended to exclude not only “hemp” but also “hemp products.” And because “hemp” under the Hemp Advancement Act includes “hemp extracts,” the bill would, in effect, exclude hemp, hemp extract, and hemp products from the definition of marihuana in the Controlled Substances Act. Query, though, whether Section 9 would unambiguously exclude “in-process hemp extract” from the definition of “marihuana” as well?
“Tetrahydrocannabinol” in the CSA’s list of schedule I substances is amended to exclude “in-process hemp extract” and “hemp products”:
Combined with the 2018 Farm Bill’s exclusion of “hemp,” this amendment would clarify that schedule I THC does not include hemp (which includes “hemp extract”) “in-process hemp extract,” or “hemp products.”
These provisions are problematic for two reasons. First, by excluding “in-process hemp extract” from the schedule I listing of “Tetrahydrocannabinol,” but not the CSA’s definition of “marihuana,” these amendments would leave open the possibility that in-process hemp extract might be treated as schedule I “marihuana.”
Second, the amendments to schedule I THC mistakenly assume that THC in schedule I includes synthetic and natural THC when in fact it includes only synthetic THC. See Hemp Indus. Ass’n v. DEA, 333 F.3d 1082 (9th Cir. 2003); Hemp Indus. Ass’n v. DEA, 357 F.3d 1012 (9th Cir. 2004). The Ninth Circuit has reversed DEA’s improper attempts to subject natural THC to schedule I controls twice before—once in 2003 and then again in 2004. Id. DEA has even admitted (albeit in informal guidance not published in the Federal Register) that its regulation defining schedule I Tetrahydrocannabinols to include natural THC is invalid. See DEA, Diversion Control Division, Internal Directive Regarding the Presence of Cannabinoids in Products and Materials Made from the Cannabis Plant (May 22, 2018)
Yet, mysteriously, DEA has never gotten around to amending its unlawful regulation:
This is no minor oversight. As the Hemp Advancement Act demonstrates, lawmakers simply assume that DEA’s regulations reflect an accurate statement of the law. As a result, they take DEA’s word for it that THC in schedule I of the CSA includes natural THC—effectively overruling the Ninth Circuits decisions to the contrary. And that is by no means the worst part.
The worst part is that the U.S. Sentencing Commission and other federal courts have also taken it for granted that DEA’s regulation reflects an accurate statement of the law. As a result, the Sentencing Guidelines’ Drug Equivalency Table for Schedule I Marijuana equates one gram of a mixture or substance containing a detectable amount of natural or synthetic tetrahydrocannabinols to 167 grams of marijuana. See U.S. Sentencing Guidelines Manual § 2D1.1, cmt. at 8(D) (U.S. Sentencing Comm’n 2021); see also id. § 2D1.1(c), (Notes to Drug Quantity Table (A)). Simply put, DEA’s refusal to amend its unlawful regulation gives prosecutors (at the state and federal levels) the ability to subject any “marihuana” violator to exponentially harsher criminal penalties simply by treating their “marihuana” violation as a “natural THC” violation.
That’s precisely what happened to Le’Ann Koss. See United States v. Koss, 812 F.3d 460 (5th Cir. 2016); see also United States v. Koss, 831 F.3d 259 (5th Cir. 2016) (Dennis, J., dissenting from denial of rehearing en banc). Because police treated the marijuana-laced substances at issue in her case as natural THC instead of “marihuana,” she was sentenced to 70 months in prison instead of 18. Koss, 831 F.3d at 260.
Koss objected to that sentence because, among other things, “neither [federal] statutes nor the Sentencing Guidelines provide any qualifying definition for THC (synthetic or organic) or any direction on how to apply its ratio provisions.” Koss, 812 F.3d at 466-467. In rejecting that argument, the Fifth Circuit relied expressly on DEA’s unlawful regulation: “Contrary to [Koss’s] assertion, the Code of Federal Regulations defines the term THC in detail. See 21 C.F.R. § 1308.11(d)(31).” Id. at 467 (emphasis added).
As the Ninth Circuit has held twice before, the THC listed in schedule I is only synthetic THC. If natural THC were already on schedule I, there would have been no reason for Congress to list “marihuana” separately. After all, all cannabis plants contain some natural THC. Hemp Indus. Ass’n, 333 F.3d at 1090 (explaining that DEA’s interpretation of tetrahydrocannabinols to include natural and synthetic THC failed because it“render[ed] superfluous the separate listing of marijuana and would nullify the explicit exemption of hemp seed and oil from the coverage of marijuana”). DEA’s failure to correct its own error over the last twenty years has caused who knows how many miscarriages of justice like the one in Le’Ann Koss’s case. The Hemp Advancement Act overlooks DEA’s egregious error and would allow the injustice to continue unchecked.
Congress obviously shouldn’t let that happen. Problem is, few if any lawmakers are even aware of the problem. Here’s hoping Congress figures it out before this situation gets even worse.